1.1 Biochemistry as a Chemical Science
Figure 1.3: Nutrients Essential for Life
Life depends on several elements to survive - in particular: carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorous. However, many other elements have other essential functions too (e.g., iron permits hemoglobin to carry oxygen).
Figure 1.4: Various Organic Compounds
The four most abundant elements in living organisms are hydrogen, oxygen, carbon, and nitrogen. Together, these four elements make up about 92% of a cells’ dry weight (as witnessed in figure 1.4).
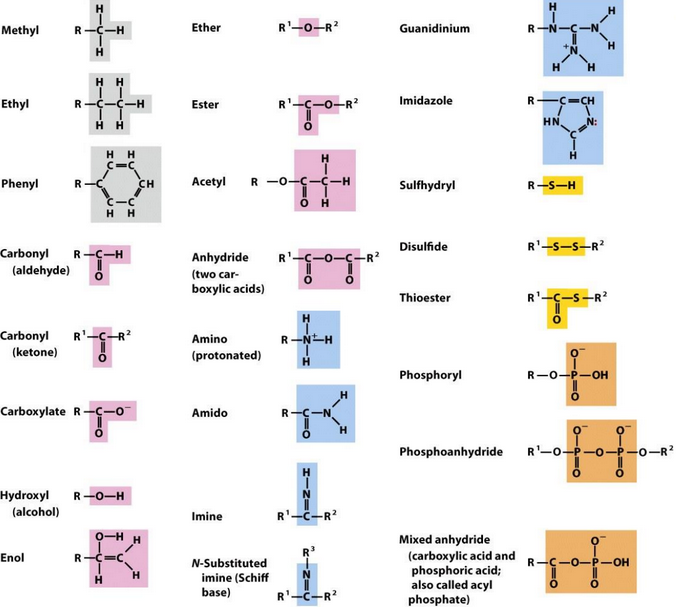
Figure 1.5: Common Functional Groups in Biomolecules
Some functional groups of biomolecules are also shown in figure 1.5. Note that the functional group of a biomolecule determines its chemical properties!