10.4 Correlative Light and Electron Microscopy
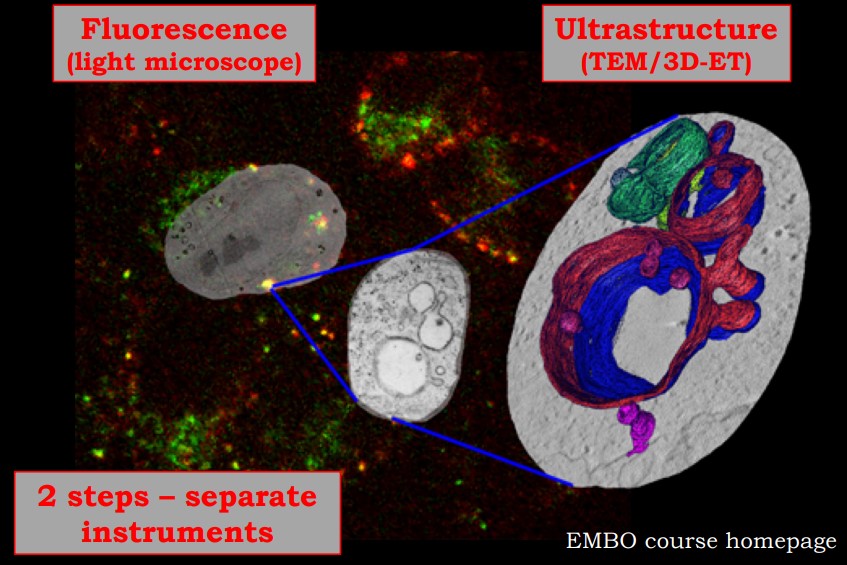
Figure 10.12: Images from a Sample CLEM
Correlative Light Electron Microscopy (i.e., CLEM) is the process of using light and electron microscopy techniques in a single experiment. CLEM can be done with any light and electron microscope.
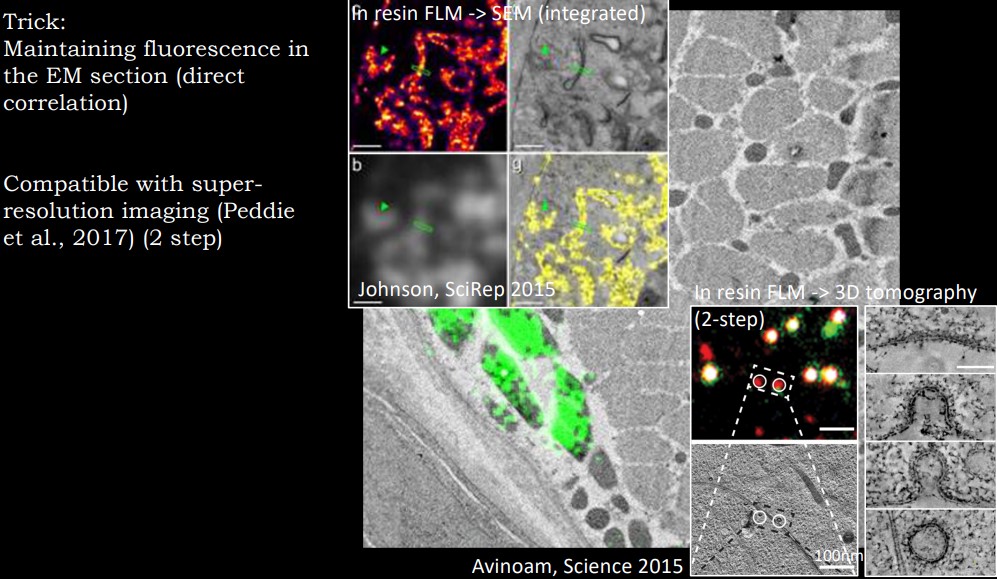
Figure 10.13: More Sample Images from a CLEM
Light microscopy give a large field of view; multi-color labeling allows one to study the dynamics of cellular processes. Electron microscopy provides higher resolution and structural information in the cellular context.
There are also two kinds of CLEM:
Conventional “two-step” CLEM
Light and electron microscopy are done on two instruments. Correlating data from both microscopes’ data are done afterwards (often with or without the help of fiducial markers5).
Integrated CLEM
Light and electron microscopy are done on the electron microscopy column; however, resolution at the light microscopy level is often limited.
10.4.1 3D TEM Imaging in Cell and Structural Biology
Three-dimensional information of protein complexes can be obtained by taking images from different angles before combining them into a 3D structure.
There are two techniques in this section:
3D electron tomography (i.e., ET)
This is used to determine larger objects (often in use for cellular imaging). Cryo ET typically uses purified viruses, organelles, or large protein complexes. The sample is vitrified (i.e., frozen) and imaged at lower temperatures.
Room temperature ET is typically used for cell or tissue sections.
Single Particle Analysis (i.e., SPA)
Here, high-resolution structures of isolated, homogeneous, and stable macromolecular complexes, viruses, and other such structures.
In a negative stain EM, the sample is left on a carbon film.
In SP Cryo EM, the object is embedded into ice and hydrated.
For instance, markers that are electron-dense and fluorescent.↩︎