6.2 FRAP, FLIP, FRET, and FLIM
6.2.1 FRAP
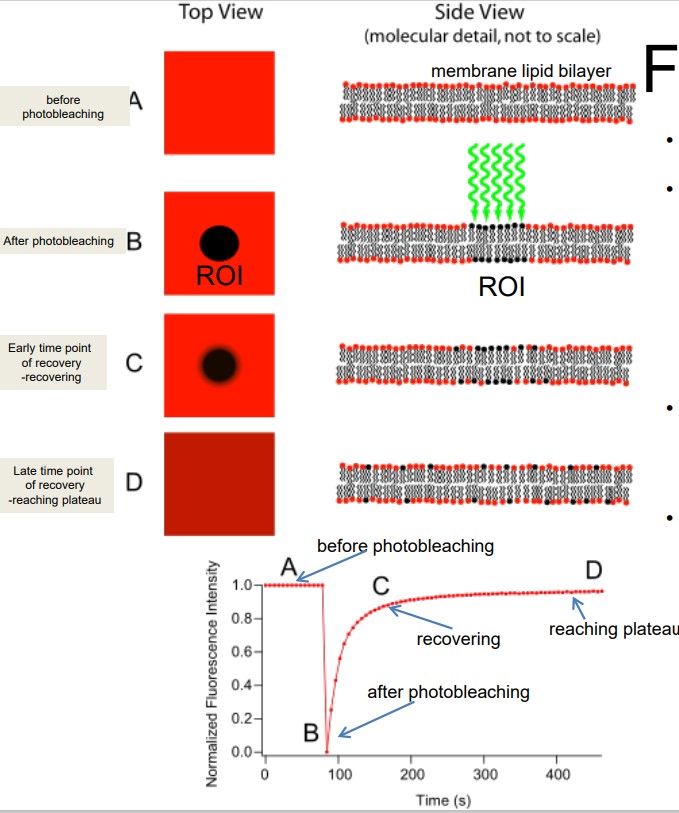
Figure 6.4: FRAP Illustrations
FRAP is short for Fluorescence recovery after photobleaching. FRAP has uses in live cell imaging and confocal microscopy (for fast and controlled photobleaching).
The general idea behind this is to label each cell component with a fluorescent molecule, image it, and photobleach that portion to observe fluorescence recovery over time. FRAP typically occurs at dynamic equilibrium conditions (i.e., photobleached and fluorescent molecules have the same biochemical properties).
FRAP has two kinds of applications outlined in BS2010:
Quantitative
This measures the kinetics of diffusion of molecules in living cells.
Qualitative
This determines the continuity of membrane organelles.
6.2.2 FLIP
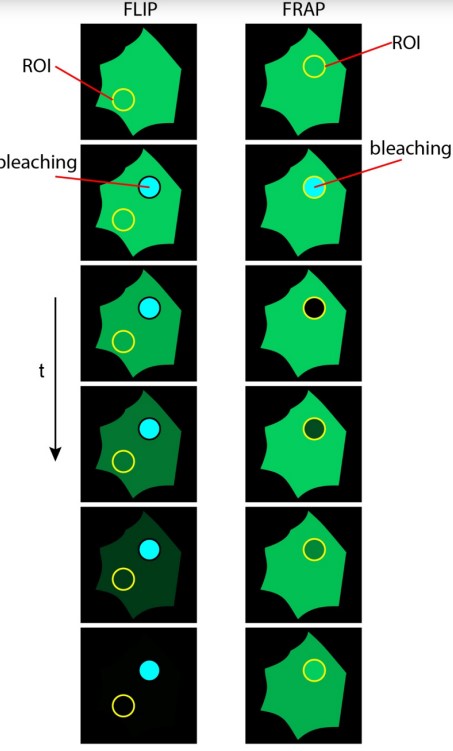
Figure 6.6: Comparison Between FLIP and FRAP
FLIP is short for fluorescence loss in photobleaching.
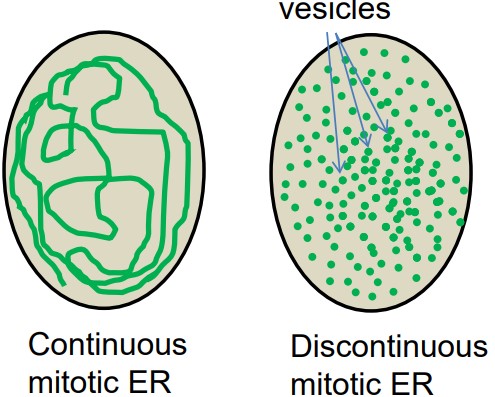
FLIP can be used to demonstrate the continuous connection of mitotic ER.
There was a debate over the status of mitotic ER - two models were initialy proposed: continuous and discontinuous.
6.2.3 FRET
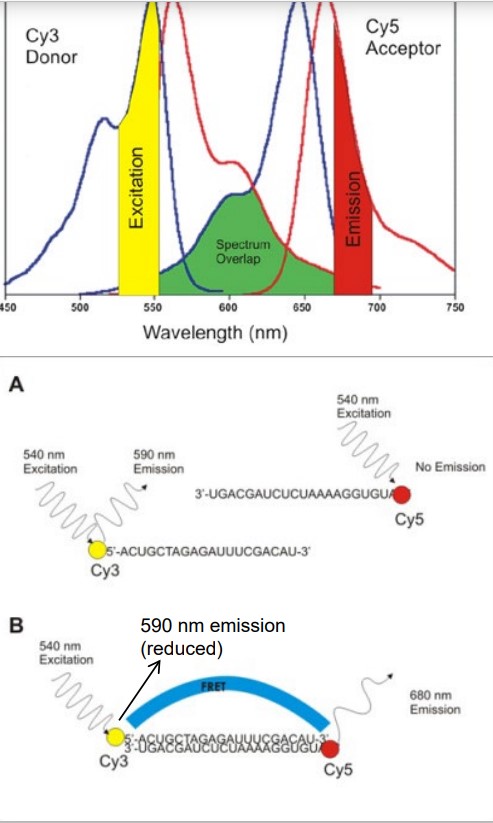
Figure 6.7: Absorption and Emission Spectrums
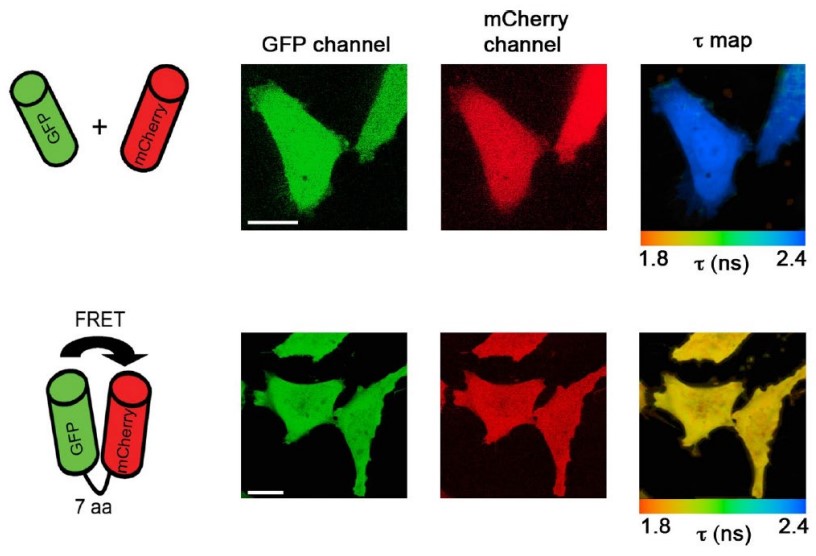
FRET is short for fluorescence (Förster) resonanceenergy transfer. This refers to radiation energy being tranferred between two fluorophores: one donor and one acceptor.
There are several conditions that need to be met in this case:
- The donor and acceptor molecules must be in close proximity.
- The absorption spectrum of the acceptor must overlap the emission spectrum of the donor.
- Both the acceptor and donor transition dipole orientations must be parallel.
FRET can be used to monitor distances or interactions between two molecules.
6.2.4 FLIM
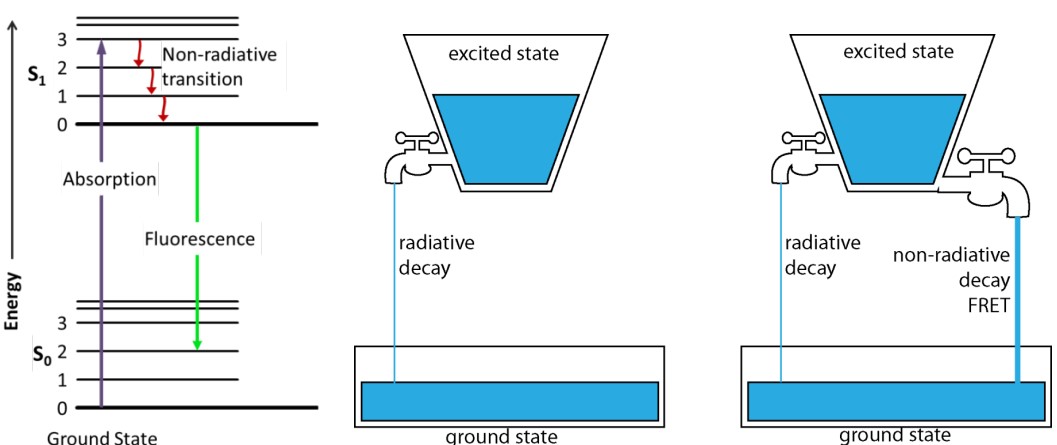
FLIM is short for fluorescence-lifetime imaging microscopy. FILM measures fluorescence lifetime.
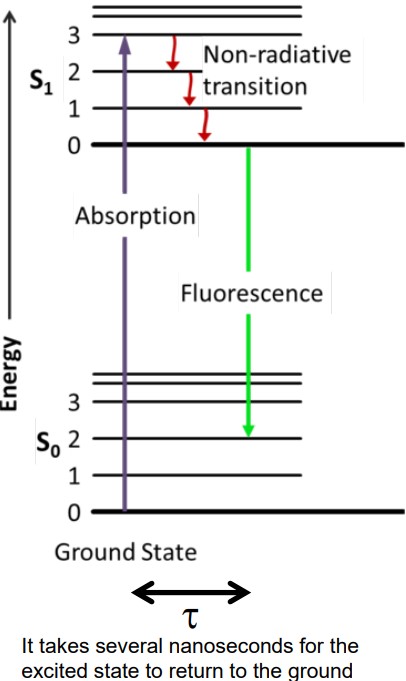
Figure 6.8: Illustration of Fluorescence Lifetime
The fluorescence lifetime τ is the residual time that a molecule (that has absorbed a photon) remains in the excited state before it comes down to its ground state by emitting a photon.
τ is sensitive to FRET, but is independent of concentration.