12.3 PET
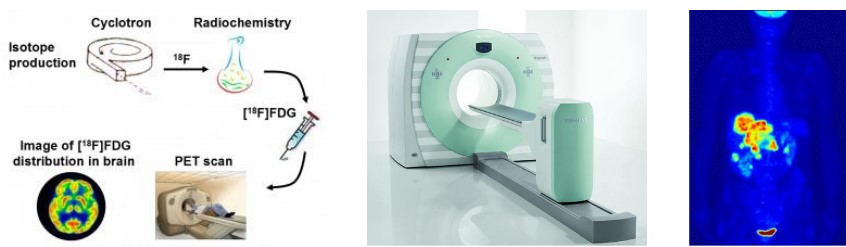
Figure 12.11: Workings of a PET scan
The Positron Emission Tomography (i.e., PET) scan is a technique that is used to observe metabolic processes in the body.
PET scans detect gamma rays from radionuclide that enters the body via Biological means.
Three dimensional images of the radionuclide’s concentrations are constructed via computers.
12.3.1 How Does a PET Scan Work?
Positron emission is used in a PET scan - this is where a nucleus degrades into a neutron and a proton.
Positrons have the same mass and charge magnitude of charge of an electron, except that the charge is positive. These are interesting objects in nuclear medicine as the main elements of life (i.e., carbon, hydrogen, oxygen, etc.) have isotopes that radiate positrons.
After a positron is generated, it scatters and collides with loosely bound electrons before fusing with one of them to form positronium (which is then annihilated). Their mass converts into energy in the form of two 511 kilo-electron volts that are emitted 180 degrees of one another.
12.3.2 How Does a PET Scan Work in Practice?
- A short-lived radioisotope is injected into the victim.
- The victim waits for the radioisotope to become concentrated in the tissues of interest.
- As the radioisotope undergoes positron emission decay, it emits a positron.
- The positron collides with a loose electron, annihilates it, and produces a pair of gamma photons that move in opposing directions.
12.3.3 Radionuclides and Radiotracers
A PET scan uses radionuclides that have a short half life. These same radionuclides are also incorporated into compounds that are normally used by the body (e.g., glucose and water). These molecules are called radiotracers.
The most common radionuclide is fluorine-18 (i.e., FDG) - it’s used in oncology and neurology. The half life of fluorine-18 is long enough that radiotracers that use fluorine-18 can be made in offsite locations and shipped to imaging centers.
12.3.4 Chelating Agents
A chelating agent is an organic compound that binds to charged metal ions to increase absorption and also has an ability to strongly bind to metal ions.
Additives can be used to form bonds to the metal ions to disrupt the chelating agent’s activity.
12.3.5 Uses of PET
Here are some scenarios that PET can be used in:
Cancer
The tracer is taken up by glucose-using cells. There is radio-labelling in tissues with high glucose uptake (e.g., brain, liver, and cancerous cells).
Neuroimaging
Radiotracers make their way into the brain via the circulatory system. Different compounds can show blood flow and oxygen and glucose metabolism in the tissues of the brain.

