4.1 What is Fluorescence?
Fluorescence is a kind of photoluminescence: a process where a kind of material absorbs photons and emits them (i.e., absorbs light as excitation energy and releases light).
Figure 4.2: Fluorescence in a Jellyfish
The Stokes shift is a phenomenon whereby the emission light of an object has a longer wavelength (i.e., the emitted photon has lesser energy).
Fluorescence typically happens in a fluorophore - a molecule that can perform fluorescence after excitation - in a few nanoseconds after light is emitted from an object. This is in contrast to phosphorescence, which typically happens minutes or hours after photons have been emitted from an object.
4.1.1 Molecular Explanation of Fluorescence
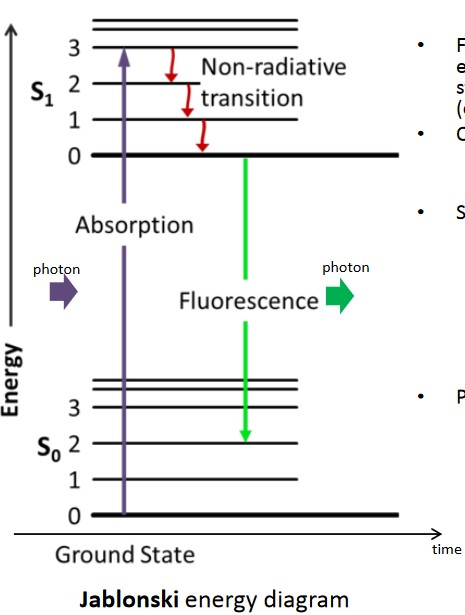
Figure 4.3: A Jablonski Energy Diagram
Fluorescence is the transition of one electron from a lower (i.e., ground state) to a higher energy level (i.e., excited state).
The quantum yield ϕ of a fluorescence is given by:
ϕ=Photons emittedPhotons absorbed
Based on a Jablonski diagram (i.e., the above graphic), the Stokes shift is a consequence of the maxima bands of the emission and absorption spectra. Emitted, fluorescent light has a lower energy (i.e., longer wavelength) than absorbed light - this is because of energy loss during the period of time that a photon is absorbed and when it is released.
Photobleaching occurs when a flurophore permanently loses its fluorescence properties due to photon-induced chemical damage.